CTSI Watch and Learn: FDA 101: A Primer on IDEs

Q-SYS Control 101: Simple Control Components (FR)Подробнее

FDA 101Подробнее

Hanwha Vision AI Application Pack - Traffic (Multilane Vehicle Counting)Подробнее

How is CDRH encouraging more US-based first-in-human studies?Подробнее

Hanwha Vision AI Application Pack - Traffic (Vehicle Speed Detection)Подробнее

FDA 101Подробнее

Technology Commercialization: The First StepsПодробнее

Building a New Medical Device (or Evolving an Existing One) with Regulations in MindПодробнее

CTSI Distinguished Speaker Series I Steven ShoptawПодробнее

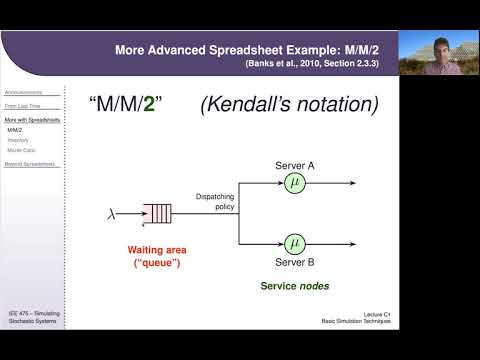
IEE 475: Lecture C1 (2020-09-10) - Basic Simulation Tools and TechniquesПодробнее
CTS Learning Series Chapter 1 – Testing Methodologies and Theory of OperationПодробнее

What Is An Investigational Device Exemption (IDE)?Подробнее

Webinar: Introduction to US Food and Drug Administration (FDA 101)Подробнее
