U.S. FDA's Unique Device Identifier (UDI) Requirements
REdI Annual Conference 2024: CDRH (Devices) Innovation in Medical Product Development (Day 2 of 2)Подробнее

Implementing a Global Unique Device Identification (UDI) Solution: Regional Update and RequirementsПодробнее

APACMed Regulatory Capacity Building Unique Device Identifier UDI Module 2Подробнее

LinkedIn Replay - How to place your UDI on Medical DevicesПодробнее

US FDA Class I UDI Requirements and ExceptionsПодробнее

Know Your Medical Device Why the Unique Device Identification UDI System Should Matter to YouПодробнее

SYS-039 UDI Requirements ProcedureПодробнее

U.S. FDA's Unique Device Identifier (UDI) RequirementsПодробнее

UDI in the EU MDR – How different is it from the US FDA?Подробнее

Tissue and Implant Tracking Requirements in HealthcareПодробнее

Direct Part Marking for FDA UDI ComplianceПодробнее

GUDID submission for FDAПодробнее

Introduction to the UDI System in the USAПодробнее

WEBINAR: Understanding the Basics of Unique Device Identification (UDI)Подробнее

FDA UDI Regulation’s Impact on Medical Device Labelers WebinarПодробнее
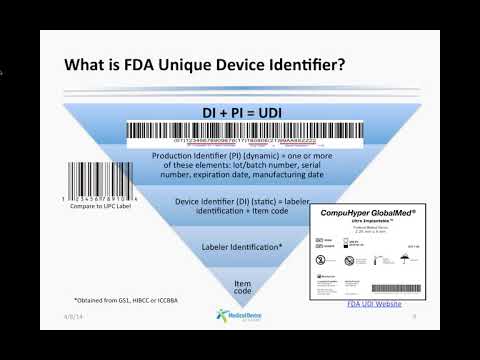
FDA UDI Marking: Are you prepared?Подробнее

Meeting the Requirements of the UDI Final Rule, an FDA PerspectiveПодробнее

Understanding the basics of Unique Device Identification (UDI)Подробнее

Advisor Live Webinar: Unique Device Identification: Implications for Hospitals & Health SystemsПодробнее

FDA 101 for Medical DevicesПодробнее
